Detecting low level radioactivity from foods

Typically a scintillation counter is used to check food(s) for radioactive contamination. The scintillation counter not only provides a radiation level in addition it provides a gamma ray spectroscopy for radioisotope identification and therefore can determine the safety of the food.
While our geiger counter can not perform radioactive identification, it may be used to test if a material or food stuff has a radioactivity above normal background radiation. This is not a safety check for food. Why? Because some radioactive contamination may be hidden in the normal fluctations of background radiation. And as we will see, while KCl has a radioactivity level well above background radiation it is commonly sold over the counter in supermarkets across the country as a salt substitute for people whom are sodium sensitive.
Performing the Experiment:
Step 1) Begin by taking a set of background radiation readings. Set GCA-07 or GCA-07W to CPM mode using the front panel switch. Log a minimum of ten one-minute readings. If you are using the radiation graphing program included with the CGA-07(W) start the program. Set the time increment to one minute. The chart below shows background readings for 60 minutes.
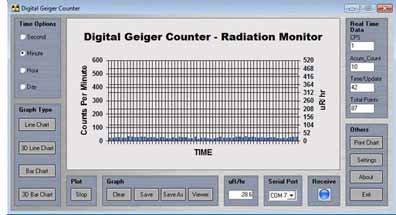
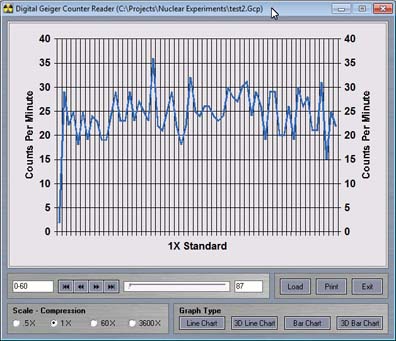
The readings may be a bit difficult to see in the standard chart program. The data may be saved, and then uploaded into the viewer program
Images Radiation Graphing program allows you to save data and to reload it into its own data viewer program.
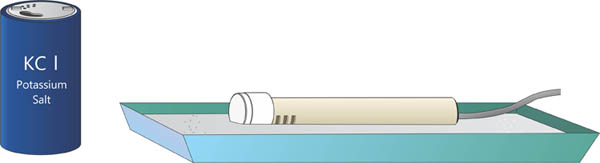
Next we take the wand and place it close to the KCl salt. You can lay the wand flat in a tray filled with salt as shown below.
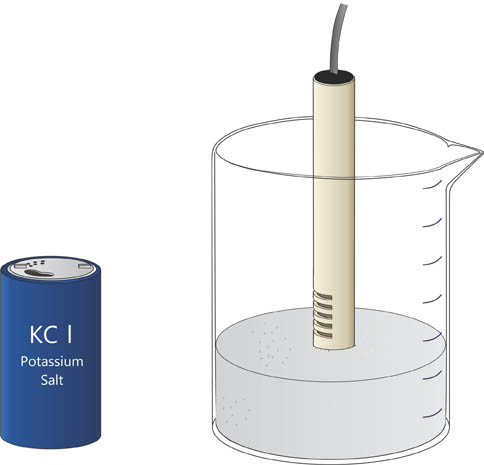
Alternatively you can also place wand in a beaker filled with KCl salt, as shown below.
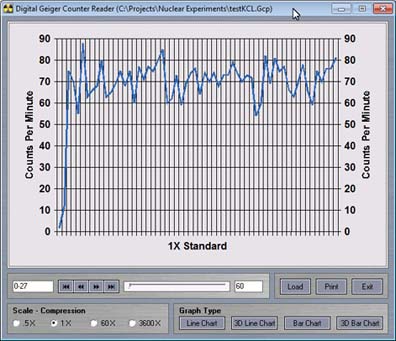
The radiation readings have more than doubled in comparison to the background radiation readings.
Again we can save the data and open it up in the viewer program.
Potassium-40 (K-40) is a naturally occuring radioactive isotope. Natural potassium contains 0.0118% of the K-40 isotope The K40 isotope has a half life of 1.28 billion years. Output radiation consisting of 89.3% beta (mean energy of 560 keV and beta maximum energy is 1.31 MeV) and 10.7% gamma with 1.461 MeV.
This information may be used for further experiments.
1) Confirm that the excessive radiation detected from the KCl does not consist of alpha radiation. A simple paper sheild should suffice.
2) Confirm that approximately 89% of the excessive radiation from the KCl cocsists of beta radiation.
More About Potassium-40
Wikipedia About Potassium-40
Data from the Graphing program may be loaded into an excel spreadsheet, see picture below.
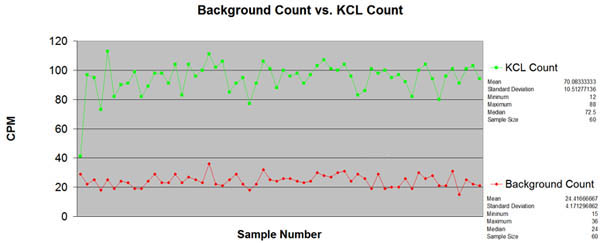
The red line shows the background radiation count and the green line details the increased radiation count from the KCl salt. Excell allows one to easily determine the Mean, Median, Min and Max CPM for the samples as shown at the side of the table. Click on the graph to obtain a higher resolution picture.


Back to Index

